The Unmet Need for Mental Health Care
Even as technological changes transform many aspects of daily life, the treatment of mental health disorders, which affect approximately 97 million people worldwide1, has remained largely unchanged for decades. Despite the shift to telemedicine due to the COVID-19 pandemic, mental health treatments are still primarily delivered in face-to-face settings and rely heavily on psychopharmaceutical interventions. Patients are also challenged by an acute lack of trained behavioral mental health clinicians and specialized treatment facilities.2 Access to behavioral health care is significantly limited by geographical location and socioeconomic factors worldwide. Meanwhile the global need for quality mental health care continues to escalate, with ever-increasing rates of depression, suicide, substance use disorders, and drug overdoses.3
Innovative, evidence-based solutions are needed to address these pressing needs. Due to the incredible leaps in technology in the past couple of decades, increasing telehealth use during the COVID-19 pandemic, and availability of new user-friendly mobile applications for most everything in our lives—including lifestyle and health care apps—there are new opportunities to marry the best of both worlds—technological and pharmaceutical—to arrive at more tailored and perhaps more convenient solutions. One of the most promising technology-driven innovations to emerge in recent years is the advent of prescription digital therapeutics (PDTs)—mobile applications that provide evidence-based behavioral therapies for a wide range of disease states and conditions and which have been developed under stringent regulatory processes. Specifically, PDTs have the potential to fill currently unmet treatment needs while expanding access to mental health care and decreasing disparities in care due to geographical or socioeconomic barriers. PDTs aim to make mental health care more scalable by reaching more patients, improving quality by standardizing treatments, reducing costs, and making healthcare more personalized by using individual patient data to individualize treatments.
What are PDTs?
PDTs are software as medical device (SaMD) treatments delivered on smartphones or tablets that address the behavioral dimensions of many diseases and conditions.4 Unlike health and wellness apps, PDTs are rigorously evaluated for safety and effectiveness in randomized controlled trials, are authorized by governmental regulatory bodies such as the U.S. Food and Drug Administration (FDA) via the device review pathways (e.g., de novo, 510K), and are subject to post-marketing requirements that are similar to regulated pharmaceuticals.5 In addition, PDTs are developed following Good Manufacturing Practices (recognized by the World Health Organization, FDA, and other groups) and include stringent security and privacy controls. PDTs are much like prescription drugs except rather than swallowing a pill or taking an injection, the treatment is delivered through software.
PDTs deliver evidence-based or scientific mechanisms of action to treat the behavioral aspects of conditions such as substance use disorders, chronic insomnia, irritable bowel syndrome, and post-traumatic stress disorder.6 PDTs have adapted well-established, evidence-based psychotherapies such as Cognitive Behavioral Therapy (CBT) for Relapse Prevention (RPT), CBT for insomnia (CBT-I), Cognitive Motivational Interviewing (MI), the Community Reinforcement Approach (CRA), and Contingency Management (CM) to digital platforms.
Behavioral treatments are widely recognized as vital components in the treatment of most mental health conditions, in part because of their significantly higher safety profile compared to pharmaceutical treatments as well as evidence that such therapies can be as effective as drugs to reduce symptoms and promote recovery.7 In the case of chronic insomnia, for example, CBT-I is the first-line treatment as recommended in recent guidelines from professional organizations in Europe,8,9 Australasia,10 and the United States.11,12 Randomized controlled trials have consistently demonstrated significant improvements in clinical outcomes and the acquisition of a range of coping and self-management skills across many of the conditions for which PDTs are currently authorized compared to standard or conventional treatments.13-15 Digital CBT treatments have also been demonstrated to be non-inferior to in-person psychotherapy alone, particularly in combination with in-person medication treatments.16,17 For example, a systematic review and meta-analysis compared electronically-delivered and face to face cognitive behavioral therapies in depressive disorders and found that digital delivery was at least as effective as face-to-face therapy.18 Moreover, CBT-based therapies can effect long-lasting, physiological and neurological changes that are indistinguishable from those induced by pharmaceuticals.19,20 For example, changes in brain connectivity have been observed following CBT in patients with depression, obsessive-compulsive disorder, post-traumatic stress disorder, and chronic pain.21 CBT for anxiety disorders is associated with changes in glucose utilization and regional cerebral blood flow.22
Multiple randomized controlled trials have demonstrated the benefits of PDTs that deliver CBT.16,23-25 In addition to the CBT-related mechanisms of action described above, PDTs may provide patients with the kinds of motivation, emotional support, and 24/7 access to supportive clinical content that no prescription drug can provide and which contribute to the overall benefits of therapy.
PDT Spotlight: Pear Therapeutics
Headquartered in the United States (Boston and San Francisco), Pear Therapeutics, Inc. was founded in 2013 to provide more inclusive and accessible software-based behavioral therapies developed with the same rigor people expect to receive from current pharmaceuticals. The goal was, and continues to be, to deliver access to much-needed treatments when and where they are needed. Pear now leads the industry in developing and delivering PDTs to patients. During the past decade, Pear has grown from a tiny start-up to a publicly-traded company with 200+ employees. It has a pipeline of products and product candidates across therapeutic areas, including the first three PDTs with disease treatment claims approved by the FDA, and the first PDT available in a second language (Spanish), which helps address issues of health equity and health care disparities. Pear has learned the importance of a flexible and agile approach to meeting various stakeholders’ needs as PDTs are integrated into practice.
Pear’s product for treating substance use disorder, reSET®, was the first PDT to receive marketing authorization from the FDA to treat disease. Pear’s second product, reSET-O®, for treating opioid use disorder, was the first PDT to receive Breakthrough Designation. Pear’s third product, Somryst®, for treating chronic insomnia, was the first PDT submitted through FDA’s traditional 510(k) pathway and became the first FDA-authorized PDT to treat chronic insomnia.26 Pear’s three PDTs have been classified by the FDA as “computerized behavioral therapy devices for psychiatric disorders.”27-29 Currently in development are PDTs for alcohol use disorder (granted Breakthrough Device Designation by the FDA in late 2021)30, major depressive disorder,31-38 and for the behavioral dimensions of additional neurology, psychiatric, and other chronic illnesses, including cardiovascular disease and cancer.39 Pear’s PDTs are now being prescribed and used by many thousands of patients across the U.S. and data from clinical trials, real-world observational studies, and health economic outcomes research clearly demonstrate the safety, clinical effectiveness, and reduced overall healthcare costs associated with PDT use.16,23-25,40,41
Pear’s PDTs from diagnosis to use
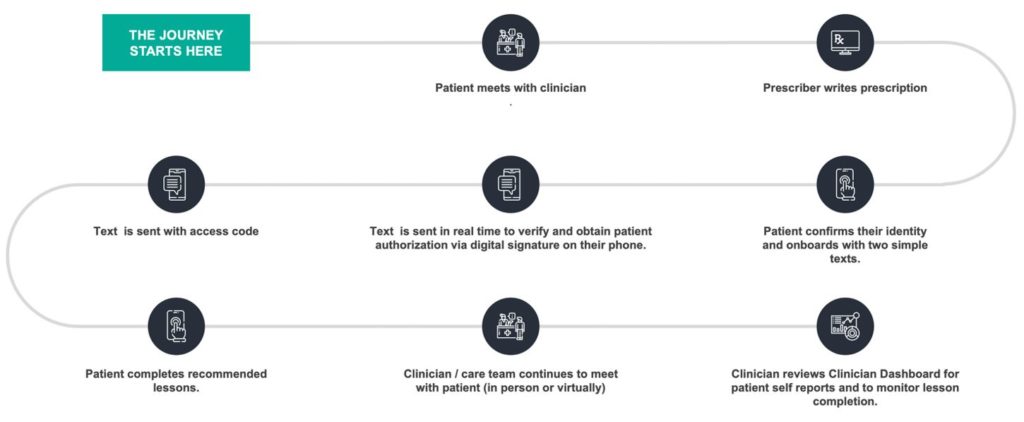
After a clinician prescribes one of Pear’s PDTs, patients are supported with downloading the therapeutic from the Apple App Store or Google Play and are guided through the subsequent activation steps. Once a password is set up, patients can begin working and learning in the PDT. The patient-facing mobile application is associated with a clinician-facing “dashboard” for monitoring and managing patient progress during the prescription (all patient data are encrypted to ensure privacy/cybersecurity).
Clinical content is conveyed to patients via a variety of digital interactions, such as sequences of self-guided interactive lessons or interactive skills practice sessions and often include video, audio, quizzes, and case-study-like vignettes. Periodic notifications can also be sent to patients to increase engagement and encourage program adherence.
Patient-reported outcomes (PROs) available via PDTs include substance cravings or triggers, symptoms, timeline follow-back data, insomnia severity index scores, and scores on other validated measures of comorbidities. In some products, patients are eligible for monetary or non-monetary motivational contingency management rewards. During the course of treatment, patients continue to see their prescribing clinician, either in-person or virtually, as needed. PDT prescriptions range from 6-12 weeks, as content and treatment duration are dependent on the disease being treated, and there are options to renew prescriptions at the discretion of the treating clinician, which is especially important in chronic conditions.
Integrating PDTs into traditional practice settings
Because clinical settings vary so widely in terms of size, organizational complexity, and the kinds of electronic health records used, the deployment and integration of PDTs are usually tailored to clinics, providers, and clinical workflows. Pear engineering, patient services, and analytics teams work closely with corresponding teams in a health care setting to ensure a smooth experience with PDTs by busy clinicians in a variety of clinical care settings. PDTs typically have specific NDC codes, just like pharmaceuticals or medical devices, which are used in existing systems for billing and insurance coverage. CPT codes also exist for clinicians to bill for their time and for monitoring adherence to treatment.
The Road Ahead
The rapid evolution of PDTs and their use to treat a range of serious mental health disorders is only going to accelerate in coming years as more companies enter the space and more PDTs are developed for a wider range of mental health conditions. In addition, technological improvements will make the programs delivered by PDTs more engaging, immersive, interactive, and effective. Mental health professionals are becoming increasingly aware of this paradigm shift in mental health treatments via organizations such as the Digital Therapeutics Alliance.
The marriage of digital health and traditional pharmaceutical treatments for mental health disorders is well underway, with the line between them growing increasingly blurry. It is likely that in the future clinicians specializing in the treatment of mental disorders will operate in a world that seamlessly blends digital and non-digital care to more effectively and precisely treat the ever-growing population of patients with acute and chronic needs for evidence-based therapies. It is critical, however, that patients with mental health disorders are treated with the highest-quality digital technologies available, therapeutics that have undergone rigorous evaluations and which have proven records of safety and effectiveness as recognized by regulatory bodies worldwide.
References
-
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789-1858.
-
Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2020 National Survey on Drug Use and Health (HHS Publication No. PEP21-07-01-003, NSDUH Series H-56). https://www.samhsa.gov/data/. Accessed October 28, 2021.
-
Substance Abuse Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2018 National Survey on Drug Use and Health. Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Adminisration;2019.
-
Food & Drug Administration. Software as a Medical Device (SaMD). https://www.fda.gov/medical-devices/digital-health-center-excellence/software-medical-device-samd. Accessed February 16, 2022.
-
Food & Drug Administration. Software as a Medical Device (SaMD): Clinical Evaluation. Guidance for Industry and Food and Drug Administration Staff. https://www.fda.gov/media/100714/download. Accessed February 16, 2022.
-
Patel NA, Butte AJ. Characteristics and challenges of the clinical pipeline of digital therapeutics. NPJ Digital Medicine. 2020;3:159.
-
Shafti SS. Pharmacotherapy vs. Psychotherapy: An Educational Challenge in Current Psychiatric Training. Scholarly Journal of Psychology and Behavioral Sciences. 2019;2(2):148-155.
-
Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675-700.
-
Wilson S, Anderson K, Baldwin D, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: An update. J Psychopharmacol. 2019;33(8):923-947.
-
Ree M, Junge M, Cunnington D. Australasian Sleep Association position statement regarding the use of psychological/behavioral treatments in the management of insomnia in adults. Sleep Med. 2017;36 Suppl 1:S43-S47.
-
Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of Chronic Insomnia Disorder in Adults: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2016;165(2):125-133.
-
Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487-504.
-
Carpenter JK, Andrews LA, Witcraft SM, Powers MB, Smits JAJ, Hofmann SG. Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depress Anxiety. 2018;35(6):502-514.
-
Ost LG, Havnen A, Hansen B, Kvale G. Cognitive behavioral treatments of obsessive-compulsive disorder. A systematic review and meta-analysis of studies published 1993-2014. Clin Psychol Rev. 2015;40:156-169.
-
Zhang A, Borhneimer LA, Weaver A, et al. Cognitive behavioral therapy for primary care depression and anxiety: a secondary meta-analytic review using robust variance estimation in meta-regression. J Behav Med. 2019;42(6):1117-1141.
-
Christensen DR, Landes RD, Jackson L, et al. Adding an internet-delivered treatment to an efficacious treatment package for opioid dependence. J Consult Clin Psychol. 2014;82(6):964-972.
-
Marsch LA, Guarino H, Acosta M, et al. Web-based behavioral treatment for substance use disorders as a partial replacement of standard methadone maintenance treatment. J Subst Abuse Treat. 2014;46(1):43-51.
-
Luo C, Sanger N, Singhal N, et al. A comparison of electronically-delivered and face to face cognitive behavioural therapies in depressive disorders: A systematic review and meta-analysis. EClinicalMedicine. 2020;24:100442.
-
Sankar A, Melin A, Lorenzetti V, Horton P, Costafreda SG, Fu CHY. A systematic review and meta-analysis of the neural correlates of psychological therapies in major depression. Psychiatry Res Neuroimaging. 2018;279:31-39.
-
Beauregard M. Functional neuroimaging studies of the effects of psychotherapy. Dialogues Clin Neurosci. 2014;16(1):75-81.
-
Pantazatos SP, Yttredahl A, Rubin-Falcone H, et al. Depression-related anterior cingulate prefrontal resting state connectivity normalizes following cognitive behavioral therapy. Eur Psychiatry. 2020;63(1):e37.
-
Brooks SJ, Stein DJ. A systematic review of the neural bases of psychotherapy for anxiety and related disorders. Dialogues Clin Neurosci. 2015;17(3):261-279.
-
Christensen H, Batterham PJ, Gosling JA, et al. Effectiveness of an online insomnia program (SHUTi) for prevention of depressive episodes (the GoodNight Study): a randomised controlled trial. Lancet Psychiatry. 2016;3(4):333-341.
-
Ritterband LM, Thorndike FP, Ingersoll KS, et al. Effect of a Web-Based Cognitive Behavior Therapy for Insomnia Intervention With 1-Year Follow-up: A Randomized Clinical Trial. JAMA Psychiatry. 2016;74(1):68-75.
-
Morin CM. Profile of Somryst Prescription Digital Therapeutic for Chronic Insomnia: Overview of Safety and Efficacy. Expert Rev Med Devices. 2020;17(12):1239-1248.
-
De Novo Classification Request for reSET (DEN160018). In: Health CfD, Radiological, eds. Washington, DC: U.S. Food & Drug Administration; 2017.
-
Food & Drug Administration. reSET-O 510k Summary. https://www.accessdata.fda.gov/cdrh_docs/pdf17/K173681.pdf. Accessed February 24, 2021.
-
Food & Drug Administration. Section 510(k) approval letter for Somryst, Computerized Behavioral Therapy Device for Pschiatric Disorders, Class II. https://www.accessdata.fda.gov/cdrh_docs/pdf19/K191716.pdf. Accessed July 14, 2020.
-
Pear Therapeutics receives FDA Breakthrough Device Designation for Prescription Digital Therapeutic candidate to treat alcohol use disorder [press release]. https://peartherapeutics.com/pear-therapeutics-receives-fda-breakthrough-device-designation-for-prescription-digital-therapeutic-candidate-to-treat-alcohol-use-disorder/, November 22, 2021.
-
Pear Therapeutics announces the acquisition of two assets addressing a comprehensive spectrum of depression conditions [press release]. https://peartherapeutics.com/pear-therapeutics-announces-the-acquisition-of-two-assets-addressing-a-comprehensive-spectrum-of-depression-conditions/, December 16, 2021.
-
Schure M, McCrory B, Tuchscherer Franklin K, Greist J, Weissman RS. Twelve-Month Follow-Up to a Fully Automated Internet-Based Cognitive Behavior Therapy Intervention for Rural Adults With Depression Symptoms: Single-Arm Longitudinal Study. J Med Internet Res. 2020;22(10):e21336.
-
Schure MB, Lindow JC, Greist JH, et al. Use of a Fully Automated Internet-Based Cognitive Behavior Therapy Intervention in a Community Population of Adults With Depression Symptoms: Randomized Controlled Trial. J Med Internet Res. 2019;21(11):e14754.
-
Whiteside U, Richards J, Steinfeld B, et al. Online cognitive behavioral therapy for depressed primary care patients: a pilot feasibility project. Perm J. 2014;18(2):21-27.
-
Marks IM, Mataix-Cols D, Kenwright M, Cameron R, Hirsch S, Gega L. Pragmatic evaluation of computer-aided self-help for anxiety and depression. Br J Psychiatry. 2003;183:57-65.
-
Osgood-Hynes DJ, Greist JH, Marks IM, et al. Self-administered psychotherapy for depression using a telephone-accessed computer system plus booklets: an open U.S.-U.K. study. J Clin Psychiatry. 1998;59(7):358-365.
-
Hollandare F, Johnsson S, Randestad M, et al. Randomized trial of Internet-based relapse prevention for partially remitted depression. Acta Psychiatr Scand. 2011;124(4):285-294.
-
Hollandare F, Anthony SA, Randestad M, et al. Two-year outcome of internet-based relapse prevention for partially remitted depression. Behav Res Ther. 2013;51(11):719-722.
-
Pear Therapeutics Inc. (US). Product Pipeline. https://peartherapeutics.com/science/product-pipeline/ Accessed February 21, 2022.
-
Velez FF, Colman S, Kauffman L, Ruetsch C, Anastassopoulos K. Real-world reduction in healthcare resource utilization following treatment of opioid use disorder with reSET-O, a novel prescription digital therapeutic. Expert Rev Pharmacoecon Outcomes Res. 2021;21(1):69-76.
-
Luderer HF, Campbell ANC, Nunes EV, et al. Engagement patterns with a digital therapeutic for substance use disorders: Correlations with abstinence outcomes. J Subst Abuse Treat. 2021:108585.






